Period 1-2 (Elements 1-10):
Happy Henry Lithely Began Baking Cakes, Not Omitting Four Necessities
Period 2-3: (Elements 11-18):
Naval Magistrates Always Signal Per Siren, Claiming Adequacy(sodium, magnesium, aluminium, silicon, phosphorus, sulphur, chlorine, argon)
Period 3-4:
(Elements 19-27):Kindly Cannibals Scare Timid Visitors, Cruelly Menacing Feeble Communist(potassium, calcium, scandium, titanium, vanadium, chromium, manganese, iron, cobalt)

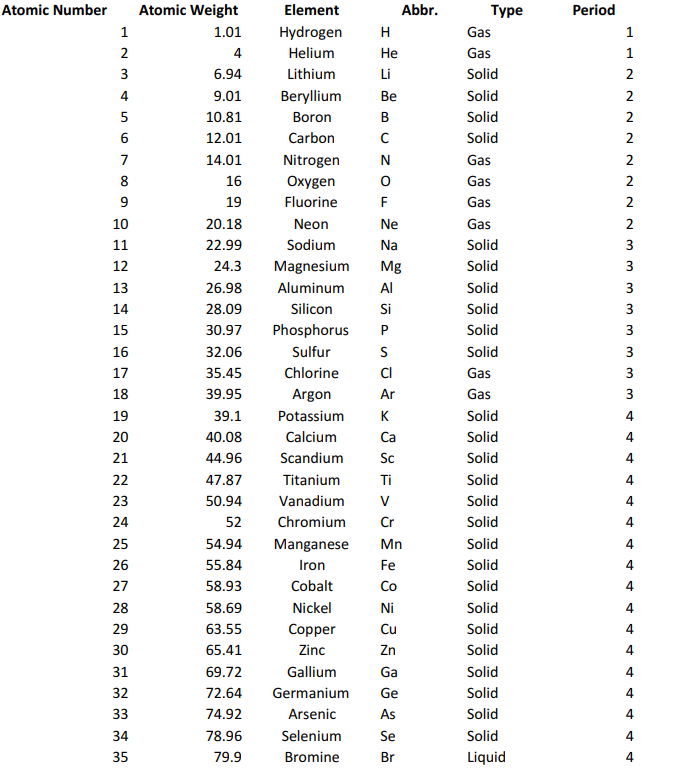
All matter consists of atoms of one or another element such as hydrogen (H) or oxygen (O), often combined into compounds (as in H2O=water). The elements are set in a Periodic Table in the order of their atomic weights (starting with hydrogen, the lightest) following Mendeleev's Periodic Law, which states that 'the properties of the elements are in periodic dependence upon their atomic weights' - in other words, elements either eight or ten places apart have many properties in common. See rare earths for the order of rare earth elements and rare gases for a mnemonic sentence governing the order of rare gases. For those completely unfamiliar with the Periodic Table itself, it is reproduced below in full.
| Jules Cesar Janssen obtained the first evidence of helium. Diagram of a helium atom. There are only two electrons orbiting helium's nucleus. Helium ballons are lighter than air. |
Helium
| Atomic Number: | 2 | Atomic Radius: | 140 pm (Van der Waals) |
| Atomic Symbol: | He | Melting Point: | -272.2 °C |
| Atomic Weight: | 4.003 | Boiling Point: | -268.93 °C |
| Electron Configuration: | 1s2 | Oxidation States: | 0 |
History
From the Greek word helios, the sun. Janssen obtained the first evidence of helium during the solar eclipse of 1868 when he detected a new line in the solar spectrum. Lockyer and Frankland suggested the name helium for the new element. In 1895 Ramsay discovered helium in the uranium mineral cleveite while it was independently discovered in cleveite by the Swedish chemists Cleve and Langlet at about the same time. Rutherford and Royds in 1907 demonstrated that alpha particles are helium nuclei.
Sources
Except for hydrogen, helium is the most abundant element found in the universe. Helium is extracted from natural gas. In fact, all natural gas contains at least trace quantities of helium.
Hydrogen Helium Periodic Table Symbol
It has been detected spectroscopically in great abundance, especially in the hotter stars, and it is an important component in both the proton-proton reaction and the carbon cycle, which account for the energy of the sun and stars.
The helium content of the atmosphere is about 1 part in 200,000. While it is present in various radioactive minerals as a decay product, the bulk of the Free World's supply is obtained from wells in Texas, Oklahoma, and Kansas. Outside the United States, the only known helium extraction plants, in 1984 were in Eastern Europe (Poland), the USSR, and a few in India.
Properties
Helium has the lowest melting point of any element and is widely used in cryogenic research because its boiling point is close to absolute zero. Also, the element is vital in the study of super conductivity.
Using liquid helium, Kurti, co-workers and others have succeeded in obtaining temperatures of a few microkelvins by the adiabatic demagnetization of copper nuclei.
Helium has other peculiar properties: It is the only liquid that cannot be solidified by lowering the temperature. It remains liquid down to absolute zero at ordinary pressures, but will readily solidify by increasing the pressure. Solid 3He and 4He are unusual in that both can be changed in volume by more than 30% by applying pressure.
Where Do Hydrogen And Helium Come From
The specific heat of helium gas is unusually high. The density of helium vapor at the normal boiling point is also very high, with the vapor expanding greatly when heated to room temperature. Containers filled with helium gas at 5 to 10 K should be treated as though they contained liquid helium due to the large increase in pressure resulting from warming the gas to room temperature.
While helium normally has a 0 valence, it seems to have a weak tendency to combine with certain other elements. Means of preparing helium difluoride have been studied, and species such as HeNe and the molecular ions He+ and He++ have been investigated.
Isotopes
Seven isotopes of helium are known: Liquid helium (He-4) exists in two forms: He-4I and He-4II, with a sharp transition point at 2.174K. He-4I (above this temperature) is a normal liquid, but He-4II (below it) is unlike any other known substance. It expands on cooling, its conductivity for heat is enormous, and neither its heat conduction nor viscosity obeys normal rules.
Uses
- as an inert gas shield for arc welding;
- a protective gas in growing silicon and germanium crystals and producing titanium and zirconium;
- as a cooling medium for nuclear reactors, and
- as a gas for supersonic wind tunnels.

Period 1-2 (Elements 1-10):
Happy Henry Lithely Began Baking Cakes, Not Omitting Four Necessities
Period 2-3: (Elements 11-18):
Naval Magistrates Always Signal Per Siren, Claiming Adequacy(sodium, magnesium, aluminium, silicon, phosphorus, sulphur, chlorine, argon)
Period 3-4:
(Elements 19-27):Kindly Cannibals Scare Timid Visitors, Cruelly Menacing Feeble Communist(potassium, calcium, scandium, titanium, vanadium, chromium, manganese, iron, cobalt)
All matter consists of atoms of one or another element such as hydrogen (H) or oxygen (O), often combined into compounds (as in H2O=water). The elements are set in a Periodic Table in the order of their atomic weights (starting with hydrogen, the lightest) following Mendeleev's Periodic Law, which states that 'the properties of the elements are in periodic dependence upon their atomic weights' - in other words, elements either eight or ten places apart have many properties in common. See rare earths for the order of rare earth elements and rare gases for a mnemonic sentence governing the order of rare gases. For those completely unfamiliar with the Periodic Table itself, it is reproduced below in full.
| Jules Cesar Janssen obtained the first evidence of helium. Diagram of a helium atom. There are only two electrons orbiting helium's nucleus. Helium ballons are lighter than air. |
Helium
| Atomic Number: | 2 | Atomic Radius: | 140 pm (Van der Waals) |
| Atomic Symbol: | He | Melting Point: | -272.2 °C |
| Atomic Weight: | 4.003 | Boiling Point: | -268.93 °C |
| Electron Configuration: | 1s2 | Oxidation States: | 0 |
History
From the Greek word helios, the sun. Janssen obtained the first evidence of helium during the solar eclipse of 1868 when he detected a new line in the solar spectrum. Lockyer and Frankland suggested the name helium for the new element. In 1895 Ramsay discovered helium in the uranium mineral cleveite while it was independently discovered in cleveite by the Swedish chemists Cleve and Langlet at about the same time. Rutherford and Royds in 1907 demonstrated that alpha particles are helium nuclei.
Sources
Except for hydrogen, helium is the most abundant element found in the universe. Helium is extracted from natural gas. In fact, all natural gas contains at least trace quantities of helium.
Hydrogen Helium Periodic Table Symbol
It has been detected spectroscopically in great abundance, especially in the hotter stars, and it is an important component in both the proton-proton reaction and the carbon cycle, which account for the energy of the sun and stars.
The helium content of the atmosphere is about 1 part in 200,000. While it is present in various radioactive minerals as a decay product, the bulk of the Free World's supply is obtained from wells in Texas, Oklahoma, and Kansas. Outside the United States, the only known helium extraction plants, in 1984 were in Eastern Europe (Poland), the USSR, and a few in India.
Properties
Helium has the lowest melting point of any element and is widely used in cryogenic research because its boiling point is close to absolute zero. Also, the element is vital in the study of super conductivity.
Using liquid helium, Kurti, co-workers and others have succeeded in obtaining temperatures of a few microkelvins by the adiabatic demagnetization of copper nuclei.
Helium has other peculiar properties: It is the only liquid that cannot be solidified by lowering the temperature. It remains liquid down to absolute zero at ordinary pressures, but will readily solidify by increasing the pressure. Solid 3He and 4He are unusual in that both can be changed in volume by more than 30% by applying pressure.
Where Do Hydrogen And Helium Come From
The specific heat of helium gas is unusually high. The density of helium vapor at the normal boiling point is also very high, with the vapor expanding greatly when heated to room temperature. Containers filled with helium gas at 5 to 10 K should be treated as though they contained liquid helium due to the large increase in pressure resulting from warming the gas to room temperature.
While helium normally has a 0 valence, it seems to have a weak tendency to combine with certain other elements. Means of preparing helium difluoride have been studied, and species such as HeNe and the molecular ions He+ and He++ have been investigated.
Isotopes
Seven isotopes of helium are known: Liquid helium (He-4) exists in two forms: He-4I and He-4II, with a sharp transition point at 2.174K. He-4I (above this temperature) is a normal liquid, but He-4II (below it) is unlike any other known substance. It expands on cooling, its conductivity for heat is enormous, and neither its heat conduction nor viscosity obeys normal rules.
Uses
- as an inert gas shield for arc welding;
- a protective gas in growing silicon and germanium crystals and producing titanium and zirconium;
- as a cooling medium for nuclear reactors, and
- as a gas for supersonic wind tunnels.
A mixture of helium and oxygen is used as an artificial atmosphere for divers and others working under pressure. Different ratios of He and O2 are used for different diver operation depths.
Helium is extensively used for filling balloons as it is a much safer gas than hydrogen. One of the recent largest uses for helium has been for pressuring liquid fuel rockets. A Saturn booster, like the type used on the Apollo lunar missions, required about 13 million ft3 of helium for a firing, plus more for checkouts.
Helium Periodic Table Of Elements
Liquid helium's use in magnetic resonance imaging (MRI) continues to increase as the medical profession accepts and develops new uses for the equipment. This equipment has eliminated some need for exploratory surgery by accurately diagnosing patients. Another medical application uses MRE to determine (by blood analysis) whether a patient has any form of cancer.
Helium is also being used to advertise on blimps for various companies, including Goodyear. Other lifting gas applications are being developed by the Navy and Air Force to detect low-flying cruise missiles. Additionally, the Drug Enforcement Agency is using radar-equipped blimps to detect drug smugglers along the United States boarders. In addition, NASA is currently using helium-filled balloons to sample the atmosphere in Antarctica to determine what is depleting the ozone layer.

